Discovery of Energy Storage Mechanisms in Electrically Conductive Coordination Frameworks
A research team consisting of researchers from NIMS, the University of Tokyo, RIKEN and the Kyoto Institute of Technology has discovered that electrically conductive coordination frameworks with tunable crystalline structures may serve as effective electrode materials for rechargeable batteries. This discovery may encourage the exploration of new types of electrode materials different from the oxide materials in current use and facilitate the development of higher-performance rechargeable batteries.
So-called “coordination materials” have been attracting of grate attention due to their tunable crystalline structures, which enable to emerge new properties and functions—such as selective molecular adsorption. Research into the use of coordination materials as electrode materials in next-generation rechargeable batteries is one of the most active research topics in present day because of its potential to enhanced energy storage properties leading to energy density. The low electrical conductivity of coordination materials, however, has hindered their application in batteries.
The NIMS-University of Tokyo research team recently hypothesized that coordination frameworks with metallic conductivity would be the key to enhance electrochemical energy storage properties involving electron transfer. In order to prove this hypothesis, a NIMS-University of Tokyo research team collaborated with a RIKEN-Kyoto Institute of Technology research team to thoroughly study the electrochemical properties of model metallically conductive coordination frameworks and investigated the crystalline structures of these materials at the world’s leading synchrotron radiation facility (SPring-8). As a result, the joint team discovered for the first time in the world that these materials are capable of storing and releasing energy by means of the insertion and de-insertion of ions driven by electrochemical reactions and that the specific capacities of these materials are comparable to those of the oxide cathode materials widely used today in rechargeable batteries.
In future studies, the joint research team plans to determine the microscopic energy storage mechanisms of conductive coordination frameworks and identify key factors for enhancing their energy storage capacities through more comprehensive investigation of the correlation between the electrochemical properties and the structures of these materials. The team expects that these studies will facilitate the formulation of guidelines for more efficient identification of promising energy storage materials.
This study was led by Ken Sakaushi (Senior Researcher, Center for Green Research on Energy and Environmental Materials, NIMS), Keisuke Wada (Doctoral student, School of Science, University of Tokyo; also a NIMS Trainee) and Hiroshi Nishihara (Professor, School of Science, University of Tokyo). This research project was supported by the NIMS Joint Research Hub Program, JSPS Grants-in-Aid for Young Scientists (B) (17K14546), JSPS Grants-in-Aid for Scientific Research (S) (26220801) and the JST-CREST project entitled, “Development of atomic or molecular two-dimensional functional films and creation of fundamental technologies for their application” (JPMJCR15F2).
This research was published in Angewandte Chemie International Edition, a journal of the German Chemical Society. Its online version was released to the public on May 28, 2018, local time.
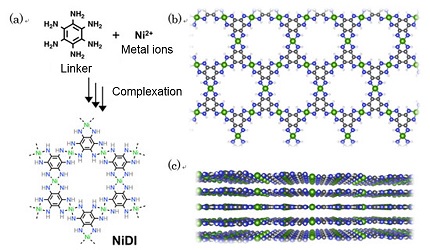
Figure. (a) Synthesis of NiDI by means of chemical reactions between organic molecules and metal ions. (b and c) Top and side views of the NiDI structure. Nickel (green), carbon (black), nitrogen (blue) and hydrogen (white). NiDI is composed of layers of atomic sheets in which molecules are arranged in hexagonal honeycomb patterns.
